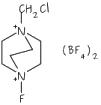Fluorinated NHC (N-heterocyclic carbene) ligands and their applications in catalysis
N-heterocyclic carbene ligands, better known as NHCs, are neutral 2-electron donor ligands that have attracted a lot of attention due to being better donors than phosphines and capable of being modified both sterically and electronically in a reasonably controlable fashion. Such ligands, and their metal complexes have been used in a number of applications, most notably as catalysts such as hydrogen-transfer reactions, and carbon-carbon bond forming reactions, but very few of them are fluorine containing ... upto now!
Fluorinated NHC ligands offer unique steric and electronic properties
Part of the attraction of these ligands comes from the ease with which their steric (size) and electronic properties may be modified. To date most of the work on NHC ligands has focussed on a few well-established examples containing either phenyl, 2,4,6-trimethylphenyl (mesityl) or 2,6-diisopropylphenyl systems. These ligands are usually known by the abbreviations IPh, IMes and IPr, and have been used to establish that NHCs are stronger donors than phosphines and that the resulting complexes are usually more stable than the phosphine analogues.
We have concentrated on making new organofluorine-containing NHC ligand systems because such ligands show a unique combination of steric and electronic properties, often unmatched from the hydrocarbon analogues listed above.
Synthesis of fluorinated NHC ligands
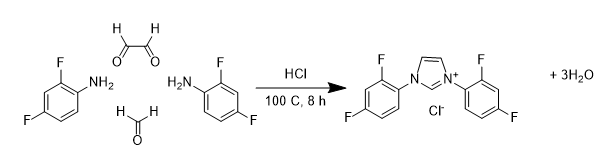
There are a number of different ways that fluorine can be introduced to NHC ligands. One approach is similar to the synthesis of non-fluorinated systems, and involves combining four different molecules including an amine that already includes fluorine (the so called building block approach) shown below:
Using this approach we have been able to make a wide range of NHC ligand precursors containing a variety of different fluorine substitution patterns on the alkyl ring. Many of these have been characterised through multi-nuclear NMR (1H, 13C, 19F) studies and often through Xray diffraction studies:
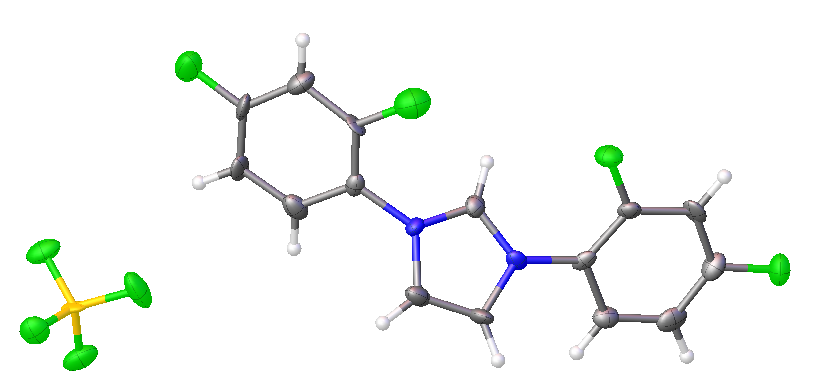
2,4-difluorophenyl NHC salt
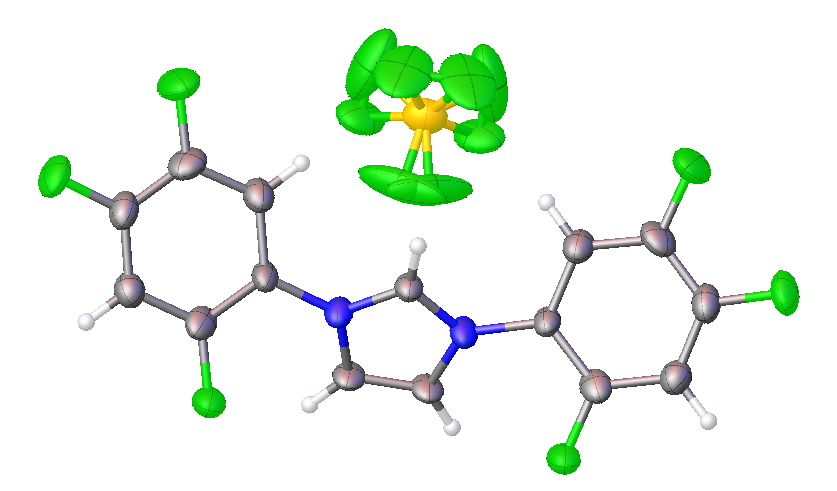
2,4,5-trifluorophenyl NHC salt
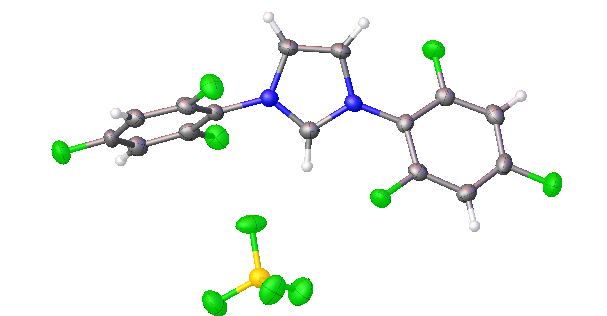
2,4,6-trifluorophenyl NHC salt
All of the above structures are of the tetrafluoroborate (BF4-) salts, rather than chlorides.
Compounds and complexes of fluorinated NHCs
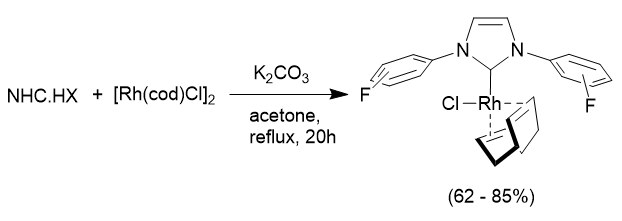
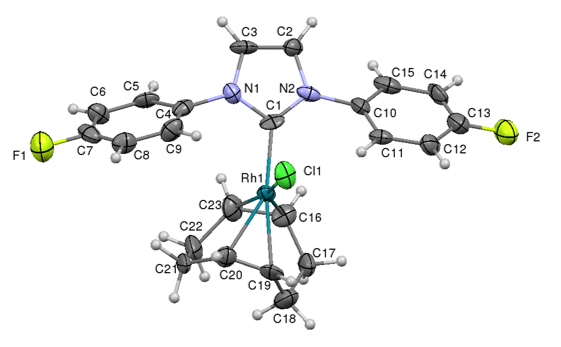
In order to assess the properties of the fluorinated NHC ligands we can use the salts and remove HX (where X is the counterion, i.e Cl- or BF4-) to generate the free carbene and coordinate this to either main group (p-block) elements, or metal complexes. In most cases synthesis is straight-forward, and two examples are illustrated below:
Properties of fluorinated NHC ligands
The compounds and complexes described above can be used to determine the steric (size) and electronic properties of the newly developed fluorinated NHC ligands.
In phosphines the substituent groups on the phosphorus donor atom point away from the metal centre so the apex angle (Tolman cone angle) of a cone that contains the entire phosphine ligand is used to determine the steric impact of the phosphine on the metal centre. However the substituents of NHCs point towards the metal to a greater extent, and so the buried volume concept has been developed (Nolan, Cavallo) which represents the percentage of the sphere of a fixed radius around a metal that is occupied by the ligand. The picture below shows these two approaches for two AuCl complexes, one containing a 4-fluorophenyl substituted phsosphine ligand and the other the 4-fluoro substituted NHC ligand.
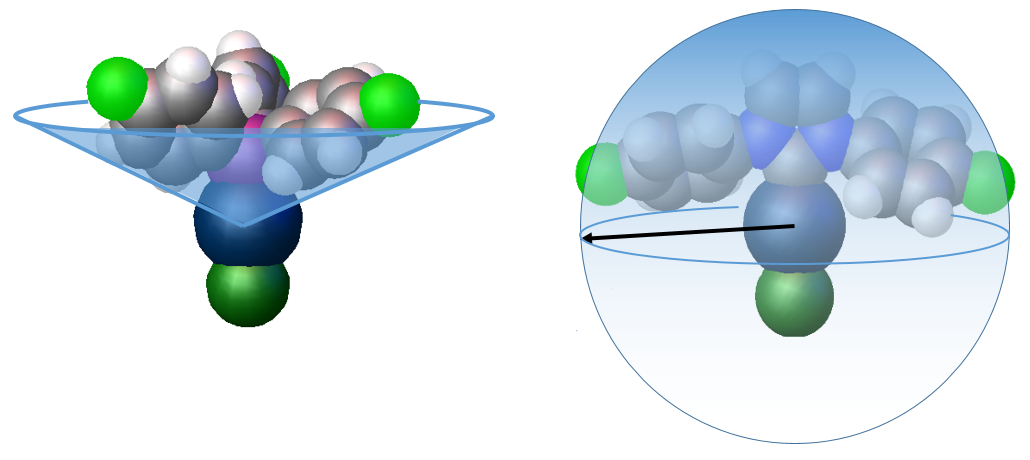
Xray structures of AuClP(C6H4F)3 illustrating the Tolman cone angle, and AuCl(4F-NHC) illustrating the buried volume steric measure
Calculations performed on the linear gold AuCl(NHC) complexes place the fluorinated NHCs at the lower end of the steric demand ranged, with %Vbur values of 30-35% (IMes by comparison is 36% and IPr 46%)
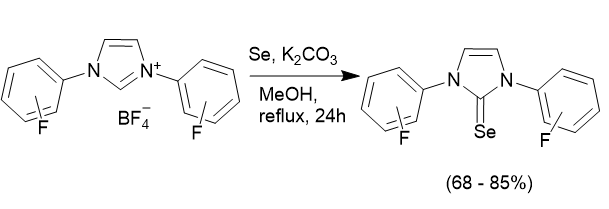
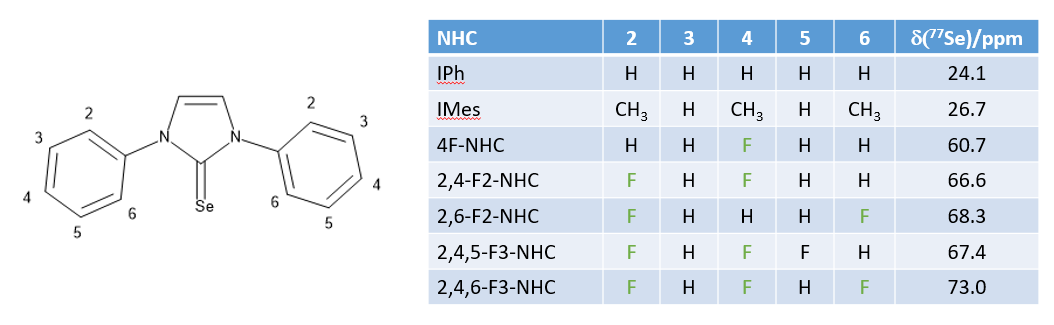
Measurements of the electronic properties of NHC ligands can be done in a number of different ways, but usually based on measuring either the IR stretching frequency, or an NMR parameter of a compound containing the NHC ligand. Using, the reaction above the selenium adducts of the fluorinated NHC ligands have been prepared and their 77Se NMR chemical shifts determined. These show that the fluorinated NHC ligands behave quite differently from the non-fluorine containing NHCs.
Fluorinated NHCs in catalysis
With a family of ligands, the steric and electronic properties of which we can modify with relative ease, but possessing distinctly different properties than those of non-fluorinated NHCs we have been investigating their use in a number of different catalytic processes.
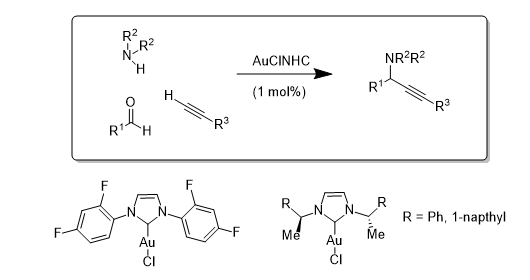
Initial work used a series of AuCl(NHC) complexes to catalyse the A3 coupling reaction [where an Aldehyde, an Amine and an Acetylene are coupled to form propargylamines]. In this case all three NHC-contaiing catalysts performed similarly:
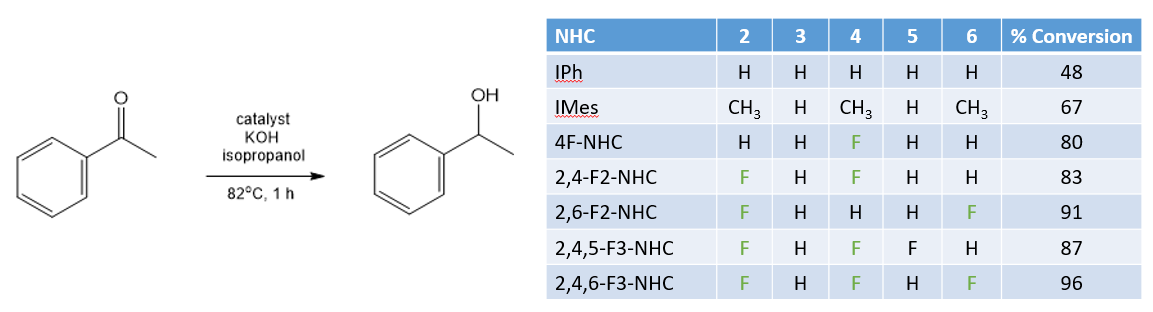
A second system studied was catalytic hydrogen transfer reactions. These involve replacing high pressure H2 with a more convenient (and less dangerous to handle) source of hydrogen. In this case the reaction used iso-propanol as the source of hydrogen and a rhodium based catalyst to transfer the hydrogen into an aldehyde:
The highest yields were obtained using fluorinated NHC ligands. Furthermore a good correlation was found between the electronic properties of the NHCs, as measured by their selenium NMR chemical shift and the conversion of the aldehyde after 1 hour:
If you would like further details of any of these areas then please contact us, details are given on the contacts page. There is also a complete list of publications available.
We greatfully acknowledge support, of various
sorts, for our research from:
![[UMIST]](/pics/crest.gif)
![]()
![]()

![]()
![]()
![]()
![]()
![]()
Cited References
A full publication list is available, as is a list of the many students and visitors who have actually generated these interesting and exciting results.

![[Pt2(S)2(PPh3)4] as predicted by our mstool The calculated mass spectrum of [Pt2(S)2(PPh3)4]](/rotator/mstool.png)