Fluoro-organometallic Chemistry
Fluoro-organometallic complexes ... new organofluorine reagents
CFCs (chlorofluorocarbons), or their bromine-containing analogues, BFCs, used to be common starting materials for the introduction of organofluorine groups into molecules or metal complexes, but these have been phased out because of the damage that they did to the ozone layer because of the presence of C-Br and C-Cl bonds in these otherwise stable and volatile compounds. Our research uses CFC-replacements as alternatives for synthetic work and as transfer agents.
Synthesis of Organometallic complexes bearing organofluorine groups
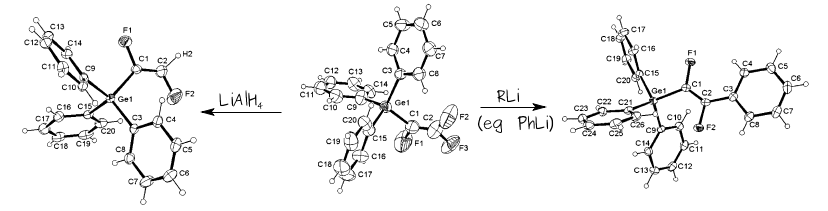
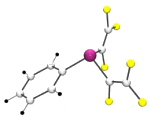
Most organometallic complexes contain hydrocarbon fragments, we seek to prepare, characterise and ultimately use, complexes which contain organofluorine moieties. Our work in the area started with the synthesis of the fluoroorganometallic lithium reagent perfluorovinyllithium, (CF2=CFLi) derived from HFC-134a (CF3CH2F).This is then used by us (and now many others use our methodology as well) as a route to a wide range of fluoroalkenyl organometallic complexes. One of these, bis(perfluorovinyl)mercury, Hg(CF=CF2)2, shown below, had the distinction of being the first metal-perfluorovinyl compound ever to be fully structurally characterised. In this case we were able to obtain the single crystal X-ray and the gas phase electron diffraction structures.Since then we have studied many more such complexes, of a wide variety of different main-group and transition-metals, such as [(C5H5)Fe(C5H4Hg(CF=CF2)] which shows an unusual pi-interaction.
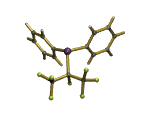
Related work showed that the chlorinated analogue CF3CH2Cl can be used to prepare 1-chloro-2,2-difluorovinyl complexes, such as the organometallic iron half-sandwich complex shown above.We were able to prepare the first examples of early-transition-metal complexes of these ligands, and ultimately to get the first crystal structure of such a complex, and evidence for a pi-bound intermediate system.
For all of these systems we are able to carry out stereospecific transformations of the fluorovinyl-group to generate trans-(CF=CFR) functions where R=H,Bu,Ph etc etc.
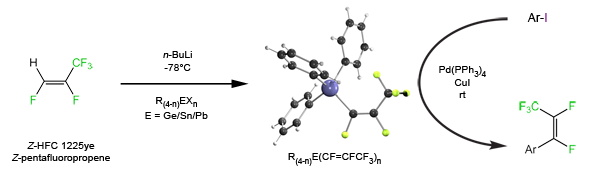
We subsequently demonstrated that other CFC-replacements may be used in a similar way. For example, hydrofluorocarbon (HFC-245fa) (CF3CHFCH2F) is capable of generating trifluoropropynyl lithium (CF3C≡CLi) under facile conditions - this route is now a method of choice for chemists.
Chemistry and Catalysis involving fluoro-organometallic complexes
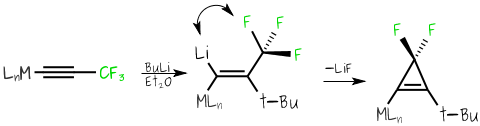
Once prepared and fully characterised we investigate the chemistry and applications of the organofluorine containing organometallic systems. For example one of the more unusual transformations that we have discovered is how we can transform a metal-bound CF3-alkyne into a difluorocyclopropene group in one single-pot reaction that relies on steric control rather than the use of toxic heavy-metal, difluorocarbene sources.Addition of a bulky base, such as t-BuLi results in close proximity of the Li and an F from the CF3 group which results in the thermodynamically favourable elimination of LiF and formation of the bound difluorocyclopropene system.
We can also use many of our systems under catalytic conditions to transfer the organofluorine group into other substrates. For example the tin pentafluoropropenyl complexes undergo Stille- Liebskind palladium-catalyzed transfer of the pentafluoropropenyl group into organic substrates.
If you would like further details of any of these areas then please contact us, details are given on the contacts page. There is also a complete list of publications available.
We greatfully acknowledge support, of various
sorts, for our research from:
![[UMIST]](/pics/crest.gif)
![]()
![]()

![]()
![]()
![]()
![]()
Cited References
A full publication list is available, as is a list of the many students and visitors who have actually generated these interesting and exciting results.

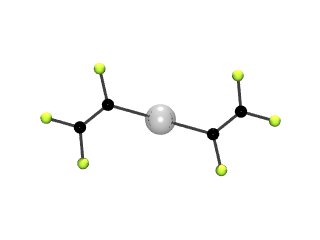
![X-ray structure of [CpFe(CO)2(CCl=CF2)] Xray structure of [CpFe(CO)2(CCl=CF2)]](/pics/fecp.jpg)