Hydrofluorocarbon (HFC) Chemistry
We use the HFCs developed to replace CFCs for new chemistry
Our research falls under the umbrella description of "Fluorine Chemistry", and we deliberately deal with aspects that straddle the usual inorganic, organometallic and organic boundaries. One area of our work, where this is obvious is the development of organofluorine chemistry of the commercially available hydrofluorocarbons (HFCs) which were developed by industry to tackle ozone depletion by CFCs and to reduce global warming as a way of introducing fluorine, or fluorinated groups into other molecules and systems.
CFC-replacements as reagents in fluorine chemistry
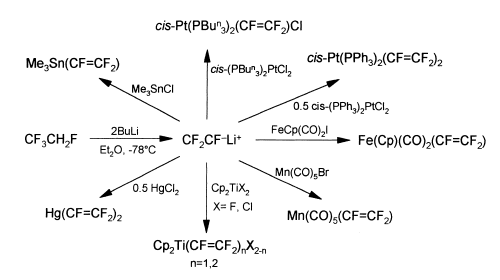
CFC's used to be common starting materials for the introduction of organo-fluorine groups into molecules, but these have been phased out because of the damage that they do to the ozone layer. We have shown that the CFC-replacments, can be used to as substitutes in chemical reactions as well. For example HFC-134a (CF3CH2F), can be used to generate perfluorovinyllithium (CF2=CFLi)
This can then be used to generate other compounds, as shown below:
Related work showed that the chlorinated analogue CF3CH2Cl can be used to prepare 1-chloro-2,2-difluorovinyl complexes.In this instance, we were able to prepare the first examples of early-transition-metal complexes of these ligands, and ultimately to get the first crystal structure of such a complex, and evidence for a pi-bound intermediate system.
For all of these systems we are able to carry out stereospecific transformations of the fluorovinyl-group to generate trans-(CF=CFR) functions where R=H,Bu,Ph etc etc.
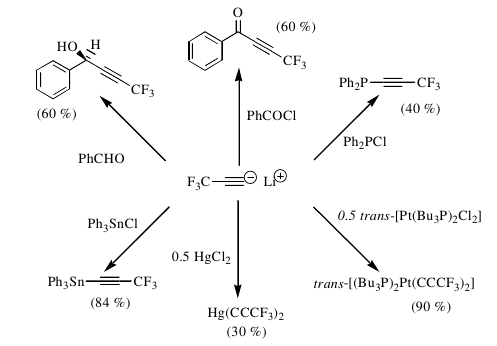
We subsequently demonstrated that a other CFC-replacements may be used in a similar way. For example, hydrofluorocarbon (HFC-245fa) (CF3CHFCH2F) is capable of generating trifluoropropynyl lithium under facile conditions.
This method is now the route of choice for many chemists, features in a number of organofluorine textbooks (for example, Modern Fluoroorganic Chemistry by Peer Kirsch, Organofluorine Chemistry by Kenji Uneyama and Fluorine in Organic Chemistry, by Dick Chambers) and can be used to generate a wide range of trifluoropropenyl-containing compounds.
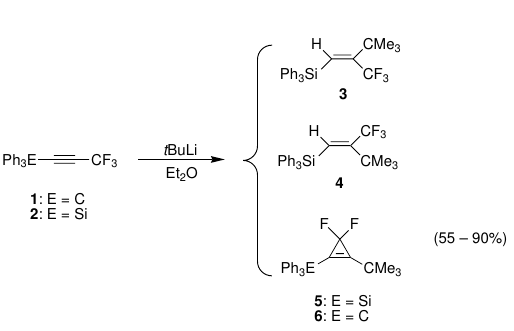
Subsequent chemistry showed that it is possible to transform some CF3-alkyne containing compounds into a difluorocyclopropene in one single-pot reaction that relies on steric control rather than the use of toxic heavy-metal, difluorocarbene sources.,
Elemental fluorine and alternatives
Traditionally fluorine is associated with high oxidation state inorganic systems - and we have published work in these areas involving the use of elemental fluorine (we have a fluorine cell in order to generate elemental fluorine electrochemically) and on alternative strategies.
We continue to seek alternative methods of introducing fluorine to molecules; this is an area in which the UMIST Fluorine group excelled, for example Professor Banks' N–F compounds such as the commercialised reagent based on F-TEDA (Selectfluor). Related to this we have recently published work on a compound bearing two N-F functionalities and we continue to look at novel electrophilic, F+, reagents.
New, simple, methods of introducing fluorine into molecules are always in demand since so many modern drugs, plant chemicals etc are fluorine-containing materials. We are investigating the catalytic introduction of fluorine using common, readily available and cheap starting materials, such as KF.
If you would like further details of any of these areas then please contact us, details are given on the contacts page. There is also a complete list of publications available.
We greatfully acknowledge support, of various
sorts, for our research from:
![[UMIST]](/pics/crest.gif)
![]()
![]()

![]()
![]()
![]()
Cited References
A full publication list is available, as is a list of the many students and visitors who have actually generated these results.