Fluorine-containing materials and applications
Fluorinated materials often possess unique properties
Fluorine-containing compounds have found a wide range of applications, ranging from modern drugs to polymeric materials. We are currently working in three applied areas of fluorine chemistry. The first of these involves the synthesis of fluoropolymer precursors, fluoropolymers and their application to the formation of well-defined architecture polymers which are recyclable. The second area also has a "green chemistry" angle - and that is the generation of new solvent systems based on fluorine-containing room-temperature ionic liquids which are being investigated from a synthetic standpoint and for their applications in electrochemistry. The third area relates to fluorination of graphene and its derivatives.
Ionic Liquids
Ionic Liquids are compounds entirely composed of ions (just like salt) but that are liquid at, or below 100 C. Room temperature ionic liquids belong to the same class of materials, but possess a melting point below room temperature. These liquids are finding industrial applications in a wide variety of areas, including as paint additives, electrolytes, extraction, recycling and gas transport.
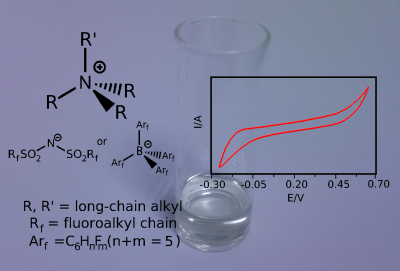
We have been working on making asymmetric ions, which pack poorly, so result in lower melting point ionic liquids, ie room temperature ionic liquids (RTILs). The systems we have prepared, based on asymmetric ammonium ions, possess low melting points (< -40C), high thermal stability, wide electrochemical windows and are insoluble in water and many organic solvents. They can be used as solvents in electrochemistry or as three-phase (triphasic) solvent systems, such as those shown below:
Fluorinated Graphene
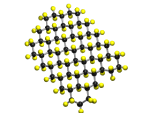
Graphene, the ultra-strong, single layer graphite-like carbon materials are being extensively studied in Manchester since our colleagues in Physics obtained the Nobel Prize for their work in that area. Our work involves modifying graphene, as this changes both the physical and electronic properties of graphene - in particular we are involved in work on fluorographene, that is layers of graphene which have been fluorinated. We have been studying both the methods of fluorination, and the properties of the resulting fluorographene materials, a representation of fluorographene is shown below, along side a real sample of fluorographene, which reults in a change in colour of graphene from grey to white.
Polymer precursors
Fluorine-containing polymers are widely used in modern society, for example for water-proofing and non-stick coatings, such as Teflon. We have been involved in EPSRC funded research on producing new functional momomers, as the building blocks for a recyclable fluorinated polymer. As such our work concentrates on devising new routes and new compounds which are appropriate monomers for subsequent polymerisation.
If you would like further details of any of these areas then please contact us, details are given on the contacts page. There is also a complete list of publications available.
We greatfully acknowledge support, of various
sorts, for our research from:
![]()
![]()
![]()
Cited References
A full publication list is available, as is a list of the many students and visitors who have actually generated these interesting and exciting results.





![Fluorous dominated structure of [AuCl{Et2P(CF=CF2)}] The crystal packing diagram of [AuCl{Et2P(CF=CF2)}]](/F_News/aurophilicity.png)

