Disclaimer and Linking Policy
While every effort has been made to ensure that the content of these web pages is accurate the author will not accept responsibility for any omissions or errors.
Any opinions expressed are those of the author, they need not coincide with those of The University.
Permission is given, by the author, to link to any of the pages on this site, provided that the link appropriately describes the content of that page.
This web-site, like most others, logs all incoming requests. These logs are stored and record information such as the page requested, the requestors computer address and browser type. No personal information is collected from visitors.
Links are provided to other sites based on those we find useful, we are not responsible for the content or operation of these other sites.
No "cookies" are generated or used by any of the web pages on this site.

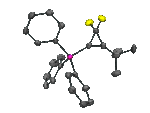
![[Pt2(S)2(PPh3)4] as predicted by our mstool The calculated mass spectrum of [Pt2(S)2(PPh3)4]](/rotator/mstool.png)